What are Process Analytic Technologies (PAT)?
Process Analytic Technologies (PAT) allow the continuous monitoring of a reaction. Traditionally, reactions were run one at a time and often sampled at a specific time point to determine reaction progress. Although this method provides accurate reaction reads, it doesn’t give us any information about how the reaction progressed to that point.
For example, a clean reaction with 80 percent conversion could exhibit any of the following characteristics and knowledge about these could lead to different strategies for further optimization.
Disadvantages of Sampling in Kinetic Profiling
Sampling is still one of the most versatile ways for kinetic profiling, but can have significant drawbacks, including:
Modern PAT methods at J-STar Research
Modern PAT are often preferred as they circumvent many of those drawbacks. J-Star Research currently uses gas uptake kinetics, in-situ IR and reaction calorimetry for very exothermic reactions. Each of those have specific applications and advantages.
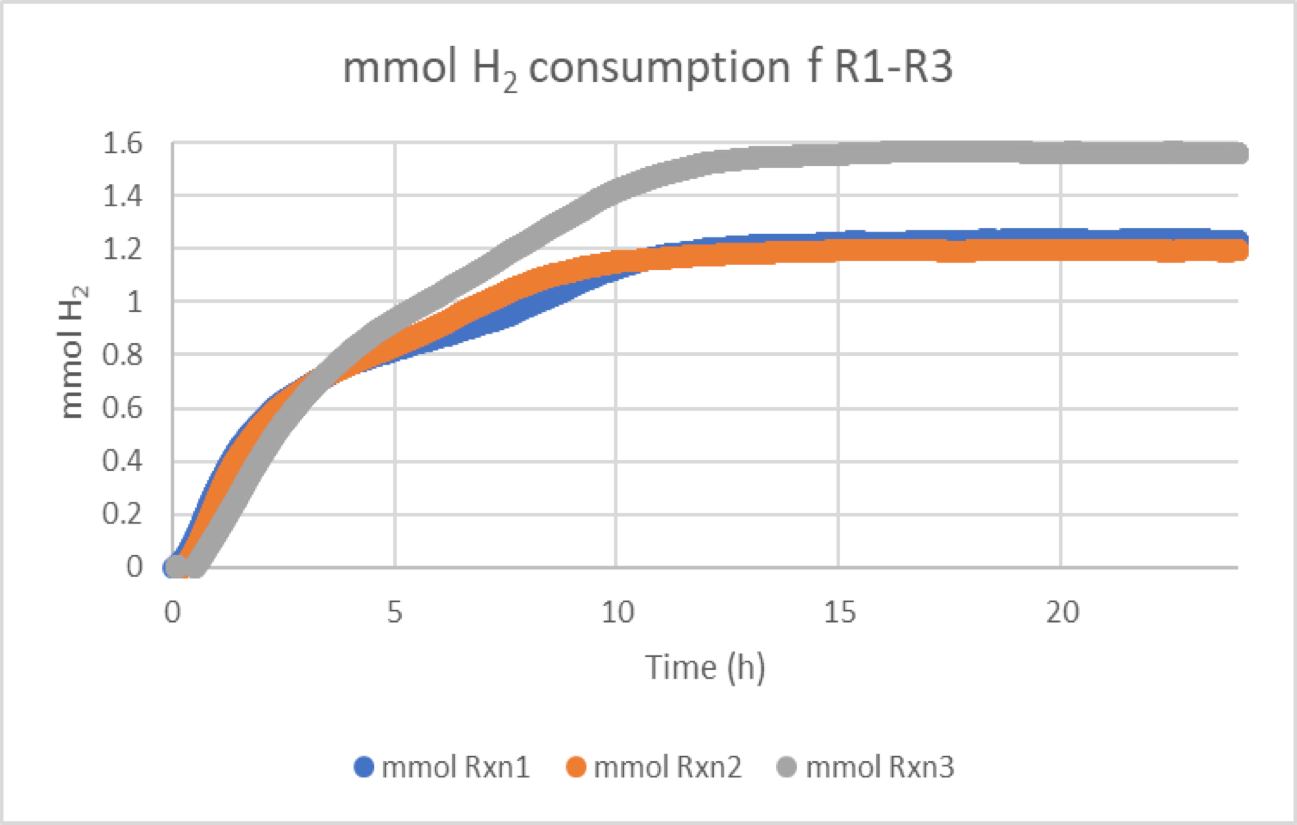
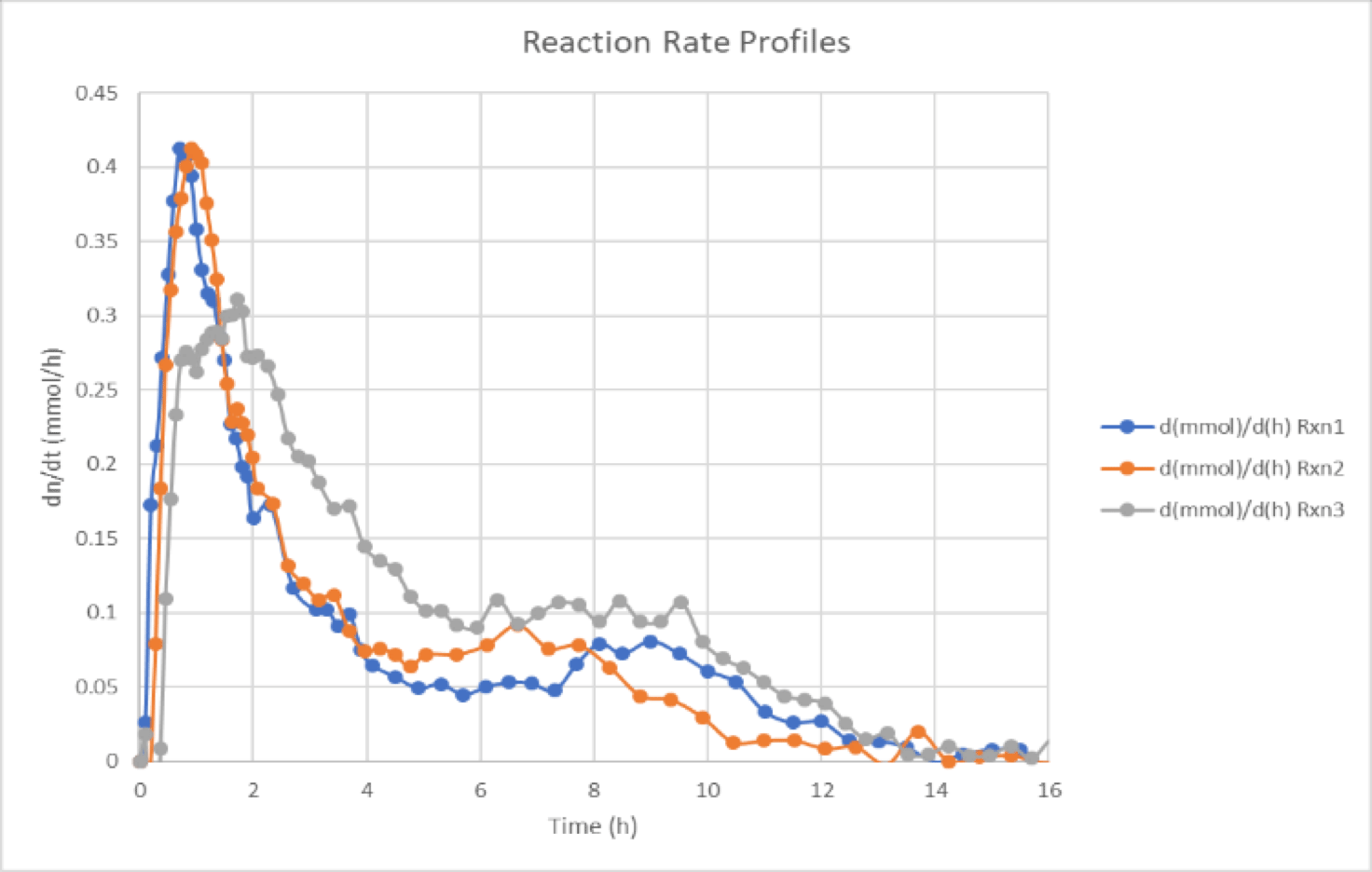
Gas uptake kinetics advantages
Gas uptake kinetics can provide significant insight, like if a hydrogenation reaction exhibits 0-order kinetics due to saturation kinetics or mass transfer limitation, a frequently observed phenomenon in poorly studied systems that can lead to impurity formation and non-scalable processes.
In-situ IR reaction monitoring
In-situ IR allows the profiling of several compounds like the substrate, product or even an intermediate that accumulates under reaction conditions. We can use IR to monitor batch and flow reactions. The In-situ IR (L) has a probe that can directly be inserted into a reaction without significantly disturbing the reaction.
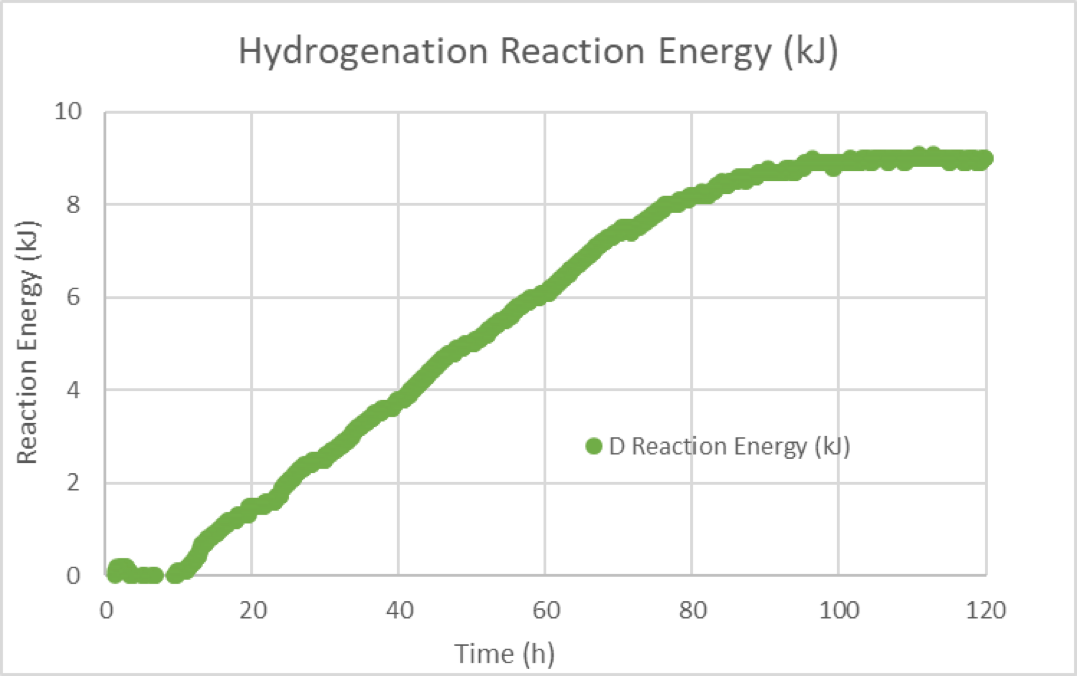
Reaction calorimetry applications
Reaction calorimetry is the only method for directly acquiring data that are proportional to the reaction rate. The other methods described above require mathematical derivatization and are therefore less favorable if the reaction rates over time need to be acquired. However, not every reaction can be monitored using reaction calorimetry. For example, the reaction needs to exhibit an exotherm or endotherm within a reasonable time scale. If the reaction enthalpy is close to ∆H = 0 or the reaction is very slow, the reaction signal will disappear in the noise.